June 13, 2010
A universal flu vaccine
Will we have a universal Influenza vaccine someday? Will we find something that eliminates the need of developing a new vaccine every year and ensuring that great part of the population receives it?
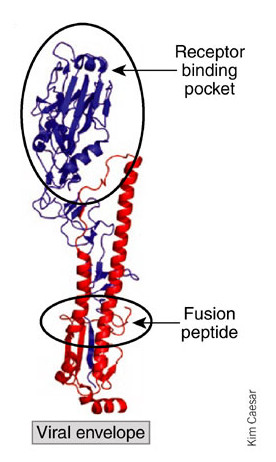
Hemagglutinin Unit. In blue, the region which is bound to the cell and in red the fusion with the membrane. Source: reference 2
Moreover, there is the worst problem: the adherence to the vaccine. Every year people need to be vaccinated and often they do not receive it. Even single dose vaccines as the one for poliomyelitis are not taken by 100% of the population.
Thus, an Influenza vaccine which ensures the protection with only a dose would be much more effective and would reduce a lot the annual costs of flu prophylaxis, with the possible benefits of protecting against variants which are not yet circulating in humans, preventing the appearing of new pandemias.
Generally, the vaccine protection is done through neutralizing antibodies. Neutralizing antibodies are those that, in addition to binding to the virus and indicate to the immune system to destroy that foreign body, are also able to avoid that the virus completes the infection successfully.
As the most immunogenic protein of the influenza virus is the Hemagglutinin, neutralizing antibodies generally hinder it of binding to sialic acid, blocking the recognition site. The recognition site is located in the surface of the HA and is promptly attacked by our antibodies. However it is a very variable region of the virus and, with some mutations of the HA, it ends not being effectively inactivated by the antibody. It happens also with the vaccination lines as we have seen previously.
Through a different approach, it was possible the production of antibodies of broad spectrum, which can recognize many Hemagglutinins of different virus. Instead of using complete viruses to develop antibodies, scientists used only the purified HA protein. In this way regions less accessible to the protein, which usually are very near to the viral membrane and unavailable, were able to be used by the immune system.
The result was really promising. Antibodies able to recognize 8 in 16 known hemagglutinins were discovered, comprising the most common antibodies in humans (and consequently more relevant), as H1 and H5. What makes this antibody able to neutralize virus as 1918’s H1N1 and avian H5N1.
The key of this achievement was the region recognized by the antibodies. Instead of the sialic acid bonding site, they attack the fusion peptide, HA region which is inserted in the cell and makes the fusion of the viral membrane with the endosome membrane, a cell structure which allows the entry of the influenza virus. As the cell membrane has specific properties, the fusion peptide needs to be much conserved and any change implies in loss of function.
Unfortunately, the H3, H7 and HAs of important viruses are not recognized by these antibodies because they belong to the second group of HAs, the group 2, which has a different fusion peptide.
The viruses recognized are all in group 1 of Hemagglutinins. On the other hand, it was not found any mutant H5N1 virus which was not neutralized by the antibodies in the tests performed, reinforcing the importance of the virus fusion region.
Studies like this, which search for new and more conserved regions of recognition by antibodies, open a perspective that one day we will have available vaccines able to combat several types of Influenza and for longer periods.
Sources:
[1] Sui, J., Hwang, W., Perez, S., Wei, G., Aird, D., Chen, L., Santelli, E., Stec, B., Cadwell, G., Ali, M., Wan, H., Murakami, A., Yammanuru, A., Han, T., Cox, N., Bankston, L., Donis, R., Liddington, R., & Marasco, W. (2009). Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses Nature Structural & Molecular Biology, 16 (3), 265-273 DOI: 10.1038/nsmb.1566
[2] Wang, T., & Palese, P. (2009). Universal epitopes of influenza virus hemagglutinins? Nature Structural & Molecular Biology, 16 (3), 233-234 DOI: 10.1038/nsmb.1574
2 Comments » Posted in: Viral structure, fighting influenza, vaccine


[...] benefits of protecting against variants which are not … Here is the original post: A universal flu vaccine « Influenza A (H1N1) Blog Posted in H1N1 Vaccination Benefits. Tags: a-lot-the, annual-costs, are-not, lot-the, much-more, [...]
[...] the recognition site. The recognition site is located in the surface of the HA … View post: A universal flu vaccine « Influenza A (H1N1) Blog Posted in H1N1 Flu, H1N1 Influenza Virus, Influenza Virus. Tags: antibodies-generally, [...]