August 27, 2009
Why do we fear Influenza 1 – the antivirals
First of all, to start this blog, and discuss the H1N1 and the flu in general, I decided to start with texts considering the other side of the Influenza. Let’s see what is going on with the virus and the decisions taken towards the virus.
![]() With viruses such as Ebola, which killed even 90% of the infected, why are we afraid of the Influenza, which usually kills less than 0.5%? Why do we think that the major pandemics are caused by a virus that may not even cause symptoms?
With viruses such as Ebola, which killed even 90% of the infected, why are we afraid of the Influenza, which usually kills less than 0.5%? Why do we think that the major pandemics are caused by a virus that may not even cause symptoms?
First of all, we have to understand why the biggest threat may be a virus, since many severe infectious diseases are caused by bacteria. Cholera, tuberculosis and others, many of which keep on killing thousands of people per year. And despite of our efforts, remain causing damages, in particular in third-world counties. However, the bacteria have a weak spot: they have a metabolism, they digest and produce molecules.
Viruses are mandatory intracellular parasites. This means that, they mandatorily must be within a cell in order to reproduce. They are incapable of performing metabolism, needing to co-opt the metabolism of the cell to them. Now, bacteria, may parasite other organisms, however, they have their own metabolism. This is the big secret of antibiotics.
We are very different from bacteria; it presents many different molecules that are attacked by antibiotics. Penicillin, for example, attacks the peptideglucanes of the bacteria cell wall. Since there is nothing similar to that in our body, penicillin may attack bacteria without affecting us.
Now, in the case of viruses, the situation is more complicated. Since they usually use our molecules, attacking them is a guarantee of severe collateral effects. A similar problem is presented when we try to fight tumors, since they are our own cells growing uncontrollably; it is difficult to attack them without causing damages to healthy cells. We need to look for something present exclusively in the viruses and attack what cannot harm us. The first targets are the enzymes.
Enzymes are proteins that catalyze chemical reactions. They speed-up reactions that would take a long time to occur. Therefore, enzymes need to be capable of connecting to what they will react, the substrate, and transform it into a product. This demands a certain format, a structure bearing the substrate and favors the reaction accelerated by it. Think in a garlic masher, that catalyzes their transformation into a paste, it needs to have a certain design and being capable of fitting the garlic in order to work. This format, such specificity is what we use against the virus.
Antiviral drugs in their majority are capable of attacking enzymes of the virus, using their specificity. Either as a substrate that cannot be broken and blocks the enzyme – an unbreakable clove of garlic in the example above – or connecting to another spot of the enzyme and inducing a change in its format that ends the specificity – as if we destroyed the axis in which the masher folds.
In the case of the HIV for example, one of the targets of the treatment is the protease of the virus, an enzyme that digests proteins, with drugs that connect and hinder them from working without attacking our proteases. Another class of drugs is the chain breakers, drugs that are incorporated by the virus polymerases, enzymes that copy the genetic material, DNA or RNA, hindering them from continuing copying. Our polymerases are less affected by such breakers since they have the function of repair. When incorporating the drug, they are capable of stopping the reaction and repairing the DNA, to the contrary of the virus.
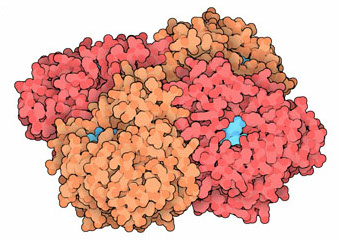
To the influenza virus, the antivirals currently available focus on two proteins. M2 forms pores that the virus uses to escape the blister through which it entered our cell, and the antiviral blocks such pore. The other protein is Neuraminidase. This enzyme cleaves our sialic acid, a sugar of the cell surface that the virus uses to enter the cell and has to cut it in order to leave it. The neuraminidase with the connected inhibitor is in the image illustrating this text.
The specificity of the enzymes is also the weakness of our fighting strategy. The bacteria are capable of escaping from antibiotics, usually destroying the drug or removing it from the cell before it exerts its effects. However, both require new steps in the metabolism, not so simple of being achieved.
Now, with respect to the viruses, it is sufficient for the enzyme to loose the affinity for the drug. With a few mutations, the virus is capable of changing its enzyme so that it loses some of its specificity. Now, the drug does not connect as it did before. This presents a cost to the virus, almost always an enzyme resistant to the drug has its activity compromised. It binds with more difficulty also to the substrate. However, a less effective enzyme is even better than an enzyme hindered from working, so that even with a certain cost, a resistant virus may be successful.
And now we search for new target enzymes, new ways to attack the virus, that hinder or at least render more difficult the selection and propagation of resistant lines. And, in particular, we have to plan the way in which we administrate current drugs in order not to lose the few resources that we have. The vaccines remain the best way to prevent and refrain Influenza epidemics.
Source:
De Clercq, E. (2002). STRATEGIES IN THE DESIGN OF ANTIVIRAL DRUGS Nature Reviews Drug Discovery, 1 (1), 13-25 DOI: 10.1038/nrd703
4 Comments » Posted in: fighting influenza


I think it’s primarily the fact that a vaccine has been so difficult to create for H1N1 that has been terrifying the public so much..
Since we’re rather close to developing an effective one, though, it shouldn’t last much longer.
The problem is that we still rely on egg produced vaccine, which depends on the creation of the hybrid virus to grow. If we already have the cell culture produced vaccine we could have started the production of vaccines as soon as we had the first sequences of the virus.
[...] In the previous text, I addressed why it is difficult to develop antivirals, here we will understand the characteristics of the influenza virus that worries us. [...]
Influenza A (H1N1) virus is a subtype of influenza A virus and the most common cause of influenza (flu) in humans. Some strains of H1N1 are endemic in humans and cause a small fraction of all influenza-like illness and a small fraction of all seasonal influenza. H1N1 strains caused a few percent all human flu infections in 2004–2005[1]. Other strains of H1N1 are endemic in pigs (swine influenza) and in birds (avian influenza).
In June 2009, the World Health Organization declared the new strain of swine-origin H1N1 as a pandemic. This strain is often called swine flu by the public media.
It’s like a serial killer that can kill anyone who is infected with it.